Proposal Preparation and Submission
Categories:
Updated: 10.24.2025
Created: 04.01.2021
Introduction
A proposal is more than a pitch document; it’s a work plan. If the agency awards the project, you will have to follow the plan that your proposal represents. Proposals are complex documents with specific requirements for each section. Be sure that you understand each component and allocate adequate time to put together your budget and gather the necessary components for your proposal. Keep in mind that some components require additional review so that should also be accounted for in your preparation timeline.
Proposal Development and Routing
Stanford internally tracks, collects, and routes all the proposal documents and approvals and waivers necessary for review and endorsement by Stanford University within the Stanford Electronic Research Administration (SeRA) System using the SeRA Proposal Development and Routing Form (PDRF).
For School of Medicine (SoM) proposals, the PI/ department should initiate, complete and route a SeRA Proposal Intake Form (PIF) to RMG for review and approval (recommended at least 30 days in advance of the sponsor deadline). The RMG Research Process Manager will follow up with a draft budget, internal deadlines, additional proposal guidance as needed, and will initiate the SeRA PDRF.
Note: the SoM SeRA PIF Process excludes SoM post doctoral fellowships and industry sponsored clinical trials.
For non-School of Medicine proposals, the PI/ department should initiate, complete and route a SeRA Proposal Development Routing Form (PDRF) to OSR for review and endorsement in accordance with the Stanford internal proposal deadline policy.
Visit the SeRA Proposal Routing webpage for complete proposal routing instructions and additional resources.
Review and Approvals
The Principal Investigator’s review and approval collected in the PDRF provides the certifications required by government agencies, and an agreement to comply with Stanford and sponsor policies.
Departmental review and approvals confirm financial commitments made in a proposal, and that stated personnel and facilities are available to carry out the project. Other required special approvals that are provided by the applicable Stanford office (such as SLAC involvement, international activity, PI waivers, etc.) are also collected and documented in the PDRF.
Final Review and Endorsement
The Institutional Official (IO) reviews the information contained in the PDRF to endorse the proposal. The IO submits the endorsed proposal to the sponsor on behalf of Stanford University.
Proposal Timeline
As you complete the PDRF, you will get a better understanding of the complexity of your proposal including the approvals that you may have to obtain from various offices on campus.

Keep in mind, reviews do not have to be sequential. If you know a proposal will require a special approval, e.g., an indirect cost waiver or waiver for PIship, initiate those requests as soon as possible. Your timeline’s starting point is the sponsor’s deadline. From there, you will factor in your institutional official’s proposal review policy (+5 business days) as well as any other special approvals you may need (see picture above). Remember to allow for extra time when the proposal includes components with external approvals such as subawards or work conducted abroad. Also don’t forget to check the timezone that your proposal is due by (PST, CT, EST, Greenwich time, etc.). Understand what is required by reading the OSR Internal Proposal Deadline Policy Memo 2015, Q&A Clarifying the University Proposal Deadline Policy and the School of Medicine Internal Proposal Deadline Policy and FAQs.
Proposal Timeline Guidance
The PI and support staff prepare the proposal in time for routing through department and school channels for approval. The complexity of your proposal and the approvals needed from other offices/departments will determine the length of your timeline.
30 days or more to prepare the proposal budget
In the School of Medicine, the PI and support staff work in close collaboration with the Research Management Group (RMG). The Research Process Manager (RPM) assigned to the applicable SoM department will also create the budget for the proposal. RMG requests a 30-day or more advance notification (School of Medicine only).
For other schools, a similar timeframe +30 days will ensure sufficient time to complete all the steps in the proposal preparation process.
In the School of Engineering, the PI and supporting staff work with the Engineering Research Administration (ERA) group to put together all the proposal documentation.
In other schools, the PI and support staff complete all of the documentation that ultimately gets routed for department/school approvals and institutional endorsement and submission.
PI Eligibility & Exceptions
Eligibility to serve as a PI or Co-PI on externally-funded research projects is a privilege generally granted only to members of the Stanford Academic Council or to the University Medical Line faculty. This policy is intended to ensure that the intellectual direction of research and scholarship is explicitly recognized as the responsibility of the PI. Designation as a project PI confers primary responsibility for the scientific, technical, and fiscal direction of the project to that individual. This designation, once granted to a specific named PI, may not be delegated to any other faculty member or staff member.
However, the University recognizes that there may be special situations for which it is acceptable to grant PI-ship to other individuals. Exceptions to the policy may be granted under special circumstances, and a waiver of PI status is required.
- For example, a researcher who is otherwise not eligible may be approved to serve as PI on externally-funded activities related to the sponsorship of conferences, exhibits, workshops, or public events; specific projects that are part of a larger interdisciplinary program; or career development awards. These exceptions must be approved by the department chair and the school dean.
- In addition, the University recognizes that there may be other unique and rare situations that warrant an exception to the policy, such as allowing short-term PI-ship for a visiting faculty member or granting permission for a not-yet-approved faculty member to submit a proposal. In addition to department and school approval, these requests must also be approved by the Dean of Research. To learn more see RPH 2.1: Principal Investigator Eligibility and Criteria for Exceptions.
- Note also that the University distinguishes between PI or co-PI and other project personnel designations (e.g., Associate Investigators), as may be needed in the presentation of specific proposal staffing requirements.
Submission of the proposal in the name of a "nominal" Principal Investigator who then delegates primary responsibility to an ineligible PI is inconsistent with the responsibility of Academic Council members for the intellectual direction of the University and is not permitted. See RPH 14.2: Academic Policies Pertaining to Sponsored Project Proposals.
Check with your school for time required to request a waiver of indirect (F&A) costs
The Dean of Research will consider requests for indirect (F&A) cost waivers in very limited circumstances. The PI should initiate the request for approval first to her department chair and school dean's office; requests must adhere to RPH . If approval is obtained, the request must be sent to the Dean of Research Office for approval.
For projects administered within the School of Medicine, the request must be sent to the Dean of the School of Medicine through the Research Management Group once it is approved by the PI’s department chair.

10 business days or more prior to sponsor deadline
The PI and School/Departmental approvals of the PDRF including attachments (at a minimum, a copy of the draft scientific portion of the proposal, internal budget and budget justification) should have been completed by now in the SeRA system. This will ensure that approvals from other offices (indirect cost waiver, global affairs review, export control review, etc.) will be completed on time for the final review and endorsement by the Institutional Official (IO). Finally, the proposal forms and documentation should be simultaneously accessible for review in the sponsor’s proposal application portal (ASSIST, Cayuse 424, Fastlane, Research.gov, etc.).
5 business days or more prior to sponsor deadline
The approvals from other offices are now complete in the PDRF (some are collected within the PDRF such as the export control review while others require for the e-mail approval to be attached to the PDRF, i.e. foundation relations approval) and the proposal is now ready for the institutional official to review. During this review period, your IO will let you know if changes or corrections are needed.
By the sponsor's deadline date and time
Once the proposal is fully compliant, the proposal is endorsed and submitted by your IO in OSR or RMG (SoM only) to the sponsor on behalf of the University via the sponsor's requested method. Remember that the proposal may be due by a set time in a different time zone from ours (e.g., MST, EST, foreign country time zone, etc..)
Check eProtocol for panel schedule
If the proposal has an extremely high probability of being awarded soon, request a protocol approval by Stanford compliance panels when the research involves human subjects, stem cells, animal subjects, or hazardous substances.
Who is My Preaward Institutional Official?
The Institutional Official (IO) is an individual named by Stanford, who is authorized to act for the institution, and to assume the obligations imposed by federal, state and local laws, regulations, requirements and conditions, as well as Stanford policy that applies to a proposal and award.
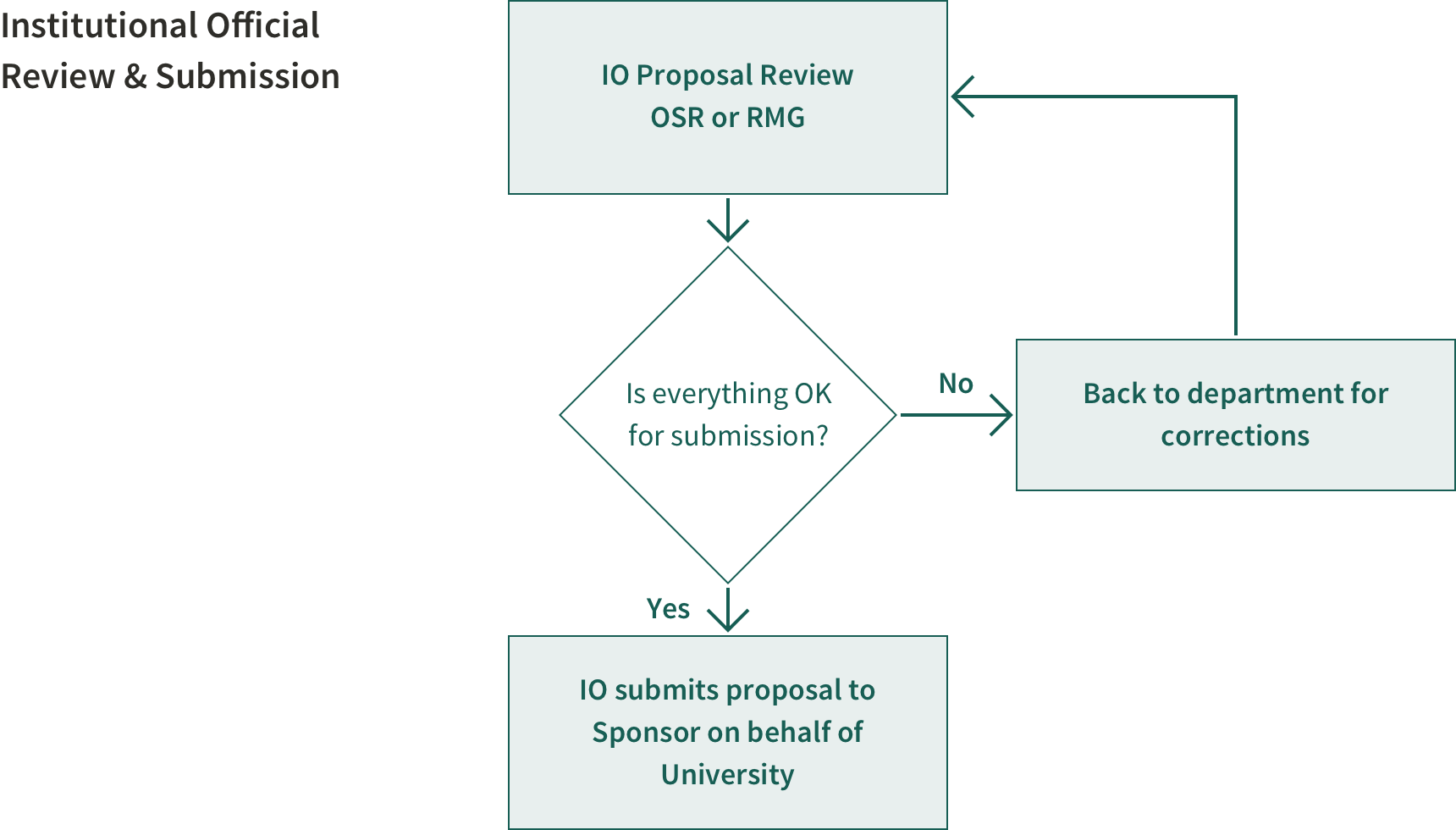
The IO reviews, endorses, signs and submits proposals to the sponsor on behalf of Stanford. In signing a proposal and in accepting a corresponding award, this individual certifies that Stanford will comply with the assurances and certifications referenced in the application.
This individual's signature further certifies that Stanford will be accountable both for appropriate use of funds awarded and performance of the sponsored project activities resulting from the application.
IO Responsibilities by Central Office
|
|
Office of Sponsored Research | Research Management Group | Industrial Contracts Office |
|---|---|---|---|
| Proposals | All Stanford University proposals except the School of Medicine | School of Medicine proposals | None |
| Awards | All awards except those handled by RMG and ICO | Federal grants, federal cooperative agreements, fellowships, and industry sponsored clinical trials for School of Medicine | Industry sponsored contracts except clinical trials |
| Subawards | All, except those under industry sponsored clinical trials | Industry sponsored clinical trials | None |
Stanford Proposal Preparation Resources
The Office of Sponsored Research, the School of Medicine's Research Management Group and the School of Engineering's Engineering Research Administration group along with your school-based research administrators can help you with your proposal.
In addition Stanford offers support for your proposals from the following offices:
Stanford Research Development Office (RDO), is a unit under VPDoR that aims to strengthen collaborative or strategic research and scholarly activities through support for funding applications. RDO supports research teams from across the University, with an emphasis on complex or strategic proposals. This often includes large, multi-PI, multi-disciplinary proposals, but can also apply to other projects depending on the discipline or specific situation.
RDO’s goal is to enhance the competitiveness of proposals through grantsmanship while reducing the burden on PIs. They provide (pre-)preaward support that might include finding the right fit between project and sponsor, supporting team formation and concept development, coordinating proposal development, and editing of proposals.
University Corporate and Foundation Relations (UCFR) is a central university office that helps to foster relationships between Stanford University and companies and private professional foundations. Part of the Office of Development, they help faculty and external funding partners connect and collaborate to advance mutual goals that align with the university’s research and teaching mission.
The Office of Science Outreach (OSO) helps faculty engage in science outreach, including organized activities targeted at youth, school teachers, and the general public that will increase their interest, understanding, and involvement in math, science, and engineering.
OSO serves faculty throughout the University by assisting them in creating outreach project ideas and proposals, identifying potential partners for them (both within Stanford as well as externally), and facilitating information and resource sharing among all of the University's science outreach programs.
They can brainstorm/suggest outreach ideas to incorporate in your proposal, review and give feedback on a draft proposal, find a specific audience/partner for your project, or write/acquire letters of support from project partners/participants. OSO also provides programs faculty members can tap into to fulfill outreach requirements while continuing to conduct research and perform teaching duties.
The Stanford Center for Clinical and Translational Education and Research (Spectrum) is an independent research center funded in part by an NIH Clinical and Translational Science Award (CTSA). Its goal is to accelerate and enhance medical research, from basic discovery to improved patient care.
Global Business Services
Are you planning travel abroad to study, research, or volunteer? Will you be collaborating with international visitors either here at Stanford or abroad? If so, you must be aware of your individual responsibilities for understanding the laws, regulations, and requirements that apply. Prepare for your international academic activity with the wealth of tools and services available to you.
University Libraries Data Management Services
Data management is emerging as a key component of funding agency requirements. Stanford University Libraries offers tools and services to help researchers comply with funding agency provisions on data management and to improve the visibility of their research.
The Data Management Planning Tool provides templates, Stanford-specific guidance, and suggested answer text for creating a data management plan for your next grant submission. The Stanford Digital Repository provides long-term preservation of your important research data in a secure, sustainable stewardship environment, combined with a persistent URL (PURL) that allows for easy data discovery, access, sharing, and reuse.
Sponsor Proposal Preparation Guidelines
Before you prepare a proposal, study and follow the current specific agency/sponsor guidelines to understand your responsibilities.
Federal agencies
Most federal agencies issue guidelines with the funding opportunities and are attached to the grants.gov listing.
The National Institutes of Health and the National Science Foundation, two of our top funders, provide many resources for proposal preparation and award management:
- NIH How to Apply
- NIH Grants Policy
- NSF Proposal and Award Policies and Procedures Guide (PAPPG) and Policy Information
Proposal Submission
Once the proposal has been reviewed by the institutional official, it gets submitted to the sponsor via the method prescribed in the associated solicitation/funding opportunity announcement. The vast majority of our proposals must be submitted via an electronic portal by an institutional official. To guide you in determining the portal to use, please see the below table:
| Sponsor | Method of submission as of 1/1/2018 |
|---|---|
| NIH |
School of Medicine: ASSIST or Cayuse Proposals (S2S) - RMG defers to the PI and Dept. re which proposal platform the PI and dept would prefer to use. All other schools: Cayuse Proposals (S2S) |
| NSF | Research.gov |
| NASA | NSPIRES or Cayuse Proposals (S2S) - OSR defers to the PI and Dept. re which proposal platform the PI and dept would prefer to use. |
| All other federal funding opportunities that prescribe using grants.gov and/or Workspace to submit an application. | Cayuse Proposals (S2S) |
| All other federal funding opportunities that prescribe using a portal OTHER THAN grants.gov and/or Workspace to submit an application. | Use the portal prescribed in the given federal funding opportunity e.g. the DoD's ebrap portal, the DoE's EERE eXCHANGE portal etc. |
| Other sponsors (foundations, industry, non-profits, State entities) | Follow application instructions in solicitation |
Federal Agencies
- Grants.gov is the official funding opportunity announcement website for the federal government. Stanford University does not use Grants.gov’s electronic proposal submission portal called Workspace. Once you locate a program announcement in Grants.gov, use the table above to determine the method of submission applicable to that program announcement.
- Cayuse Proposals (S2S) is a web-based software service that provides faculty researchers and support staff an easier, faster interface to Grants.gov for submitting research proposals to federal agencies.
- NSF Research.gov is the National Science Foundation online system that support all functions of the proposal process: submission, review, award, and reporting. All reporting functionality (technical and financial) is in Research.gov. The old NSF FastLane system has been retired. For a status update on FastLane system decommissioning and transition to Research.gov transition click Here.
- NASA NSPIRES - NASA utilizes this online system to announce NASA funding opportunities. In some instances, pre-proposals and/or full proposals are accepted via NSPIRES.
- NIH ERA Commons is an investigator registration system that works in conjunction with ASSIST and Grants.gov to insure receipt of applications by the National Institutes of Health. All investigators must be registered in NIH Commons prior to submitting proposals to NIH and other Public Health Service agencies.
Private Agencies
Proposals to foundations, corporations and other non-profit agencies are submitted via a variety of methods. Make sure to check the instructions from the sponsor and verify that we are registered for their electronic method of submission. Some foundations also require coordination and prior approval with foundation relations. Please refer to the Restricted Foundation List. Applications to restricted foundations require coordination with the Office of University Foundation Relations.
- Proposal Central supports a variety of non-profit funding agencies in proposal submission. Agencies that utilize this system include the American Cancer Society, the Arthritis Foundation, the Cystic Fibrosis Foundation, and the Muscular Dystrophy Association.
Policies
Resources
How To
- Prepare the Other Support Documentation to Submit to NIH
- Get a PI Waiver
- Access and Complete PI Training
- Use a Service Center
Training
Tools & Documents
- OSR Internal Proposal Deadline Policy
- Institutional Facts
- Faculty Effort on Sponsored Research - FAQS
- Cost Sharing FAQs