Protocols
Categories:
Updated: 08.25.2025
Created: 03.26.2021
Introduction
Approval of research protocols by the appropriate Administrative Panel is required before beginning research that involves: human subjects; recombinant DNA molecules; human stem cells; human embryos or their derivatives; laboratory animals; infectious or biohazardous agents; radioactive isotopes; or ionizing, ultraviolet laser, and/or microwave radiation.
Protocol Submission, Review and Approval
The eProtocol system is used to submit, review, and approve research protocols. The eProtocol system will guide you through the specific process for each Administrative Panel, and provides a convenient place to manage your protocols. Please see the Panel Meeting Dates & Deadlines for a complete list of protocol submission deadlines and meeting dates.
Administrative Panels
- IRB for human subjects (medical and non-medical)
- IRB/SCRO for human stem cells, human embryos, or other derivatives
- IACUC for laboratory animals
- APB for infectious or biohazardous agents and/or recombinant DNA
- APRS for radioactive isotopes or ionizing, ultraviolet laser, and/or microwave radiation
Human Subjects, Human Stem Cells, Human Embryos, or their Derivatives
Studies that meet the definition of human subject research must be submitted to the IRB and must receive IRB approval before any study activities take place. The HRPP Policy Manual, Chapter 1.4 defines human subject research (HSR) and provides detailed information about the protection program, guidelines for HSR, and training requirements. The Human & Animal Research Compliance Office (Human & Animal RCO) provides forms and checklists for both medical and non-medical protocols to determine whether and what type of review your research requires. See Does My Project Need IRB Review?
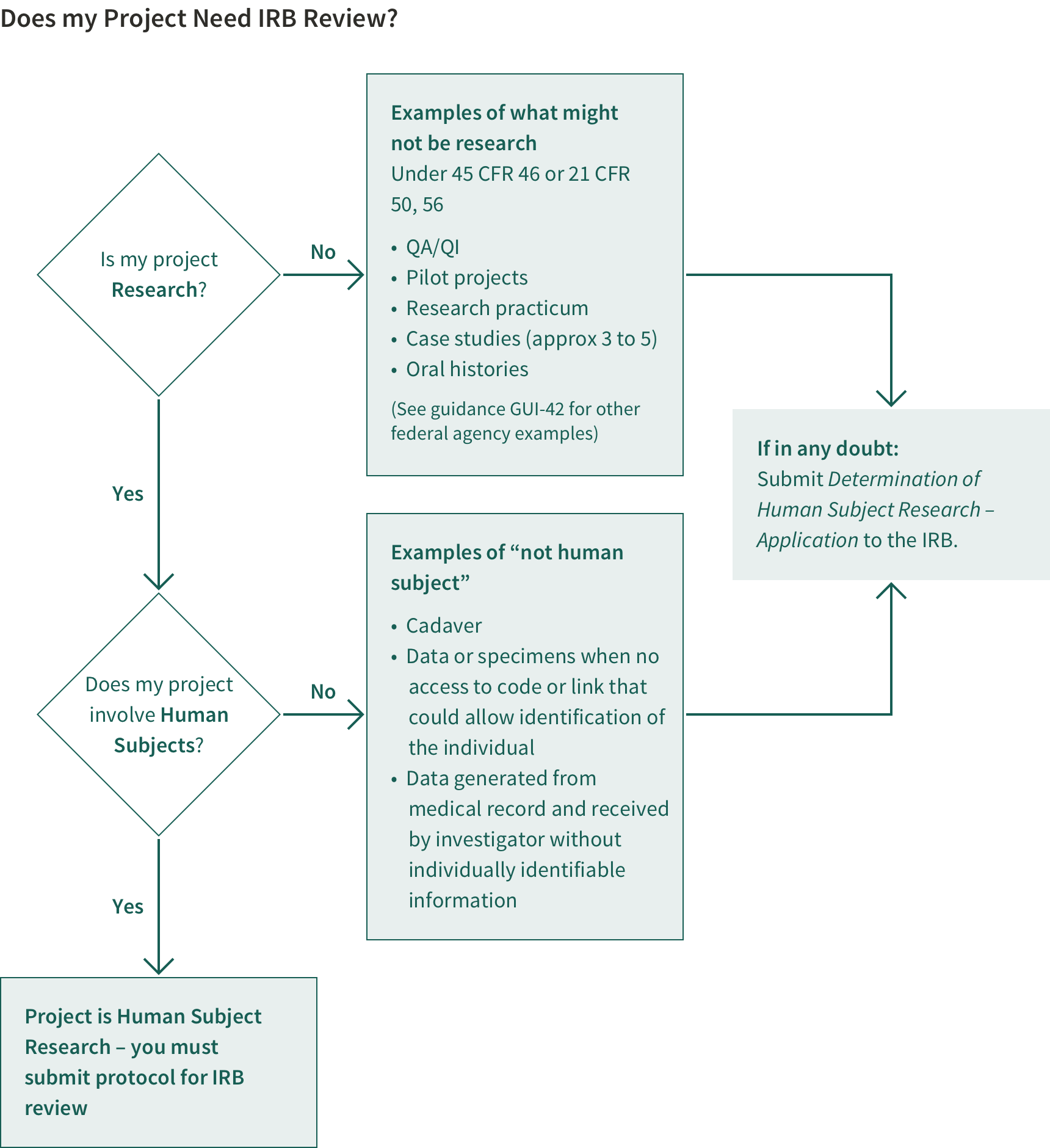
When to Submit Your Protocol
If your study is considered to be human subjects research by federal definitions, or a clinical investigation by the FDA, it must be submitted to the Stanford Institutional Review Board (IRB) and must receive IRB approval or an exempt determination before any study activities take place (if unsure, see Do I need an IRB Submission?)and before the associated grant or contract can be endorsed by the University and approved in .
The Proposal Development Routing Form (PDRF) in SeRAprompts you to disclose if human subjects will be used in the performance of the study. While the human subject research in your project might not start until well into the sponsored project’s period of performance, the award cannot be released in SeRA until a protocol is approved and the correct SPO number is listed as a funding source in the eProtocol system. You should work with the study coordinator to make sure that the correct SPO number is listed as a funding source.
A single protocol can be supported by multiple funding sources, and a single funding source can support multiple protocols. Feel free to coordinate with the appropriate IRB panel manager if you have any questions as to whether a SPO can be added as a funding source to an existing protocol, or if a new protocol must be submitted for full review. A revised protocol can be submitted to add a SPO number as a funding source, and these types of revised submissions do not need to wait for the convened meeting to be approved when there is no change in the use of human subjects.
If your project includes subrecipients, check with your Principal Investigator if their work will be subject to IRB review as well. To ensure uninterrupted processing of setting up a subaward, collect the subrecipient’s own IRB approval letter and include it as an attachment in Stanford’s IRB protocol submission. Continued review of the subrecipient’s approval is required as a part of Stanford’s compliance responsibilities. A revised protocol can be submitted to add any attachments as necessary. A subaward agreement cannot be signed by OSR until the subrecipient’s approval letter is uploaded to the Stanford protocol and the revised submission is approved by the Stanford IRB. It is recommended that IRB letters be collected and submitted for Stanford IRB approval before a subaward requisition is routed for OSR processing.
In an effort to reduce duplicative IRB review of the same protocol by multiple sites, the National Institutes of Health (NIH) requires use of a single IRB (sIRB) for multi-site studies. The other participating sites rely on the sIRB’s review and approval. In the event a Stanford study will rely on another site, a local (Stanford sIRB) protocol must be submitted to the Stanford IRB for review and approval. These studies will receive an administrative reliance letter from the Stanford IRB and must be tracked in SeRA per usual processing. See Single IRB.
While human subject research requires absolute compliance with numerous policies and regulations, the Stanford IRB and your Office of Sponsored Research (OSR) or Research Management Group (RMG) representative are here to help. IRB questions about reliance on a sIRB can be directed to singleirb@stanford.edu.
Extended Approval
For most protocols that involve no more than minimal risk to human subjects with certain exceptions (see FAQ) the IRBs permits extended approval period which does not require continuing review.
Animal Subjects
All research and teaching activities involving live or dead vertebrate animal use must be reviewed and approved by the IACUC (Institutional Animal Care and Use Committee) prior to commencement of the activity. In order to obtain approval for your activities, you will need to complete and submit an electronic protocol application form in eprotocol detailing your intended use of animals. In general, protocol applications received by the first working day of the month will be reviewed at that month’s IACUC meeting. The Laboratory Animal Occupational Health Program (LAOHP) provides information and safeguards for personnel working with laboratory animals. The program is administered through Stanford’s Department of Environmental Health & Safety (EH&S) in the onsite Stanford University Occupational Health Center (SUOHC), in close cooperation with the Department of Comparative Medicine (DCM) and the Institutional Animal Care and Use Program for Animal Care and Use (IACUP).
Infectious or Biohazardous Agents and/or Recombinant DNA
Any work using biological agents classified as BSL-2 (Biosafety 2) or above must have APB (Administrative Panel on Biosafety) approval prior to commencing work. BSL designations are based on risk determinations, as well as information available from resources such as the NIH, CDC or American Biological Safety Association (ABSA); BSL designations from commercial companies must be confirmed with Biosafety prior to use of the agent(s).
Any work using rDNA that is deemed by the NIH to be non-exempt from the rDNA Guidelines must have APB approval prior to commencing work.
To determine if your research requires APB approval, check:
- NIH rDNA Guidelines
- CDC Biosafety in Microbiological and Biomedical Laboratories (BMBL) 6th Edition
- American Biological Safety Association
- Public Health Agency of Canada Pathogen Safety Data Sheets
You can also check with the biosafety personnel at Environmental Health and Safety (EH&S) biosafety@lists.stanford.edu.
Viruses and viral vectors have become a staple of the molecular biology community. As such, it is important for users to understand the origins of these tools and potential implications of their use. The following viral vector-related documents are available:
- Working with Viral Vectors - Sections for each virus contain information on virology, clinical features, epidemiology, treatment, laboratory hazards, Personnel Protective Equipment (PPE), disinfection, and use with animals.
- Recombinant Viral Vector Biosafety Levels - Information on recombinant viral vectors and the resulting biosafety levels.
- The APB uses the eProtocol Biosafety application process, a web-based system that coordinates new protocols, updates, renewals and reminders. To learn more about eProtocol biosafety and access the system, go to eProtocol Biosafety
- Human subjects protocols involving gene transfer, gene therapy or infectious agents must be reviewed and approved by the Panel prior to initiation of protocol.
- Applications to the APB must be under the name of a faculty member
Radioactive Isotopes or Ionizing, Ultraviolet Laser, and/or Microwave Radiation
The Administrative Panel on Radiation Safety (APRS) has delegated reviews of Medical Research Protocols (MRP) to the Clinical Radiation Safety Committee (CRSCo) at Stanford University and Stanford Health Care. Any human research that involves the use of radioactive isotopes and/or radiation producing devices will require CRSCo approval.
If you are a protocol director or research coordinator for human research studies, you must use Stanford’s eProtocol system to enter the details of the study.
If your human research involves radiation (e.g. Fluoroscopy, CT, PET/CT, nuclear medicine, or DEXA scans), you will be required to complete Section 4: “Radioisotopes or Radiation Machines” and Section 9: “Risks” for all procedures that involve radiation. In parallel to the Institutional Review Board (IRB), the Health Physics - Radiation Safety Panel will review your protocol and provide a radiation dose estimate, as well as risk language for the informed consent process.
The goal of eProtocol (and our review) is to ensure that each subject is properly informed of the risks he or she may be exposed to while participating in the study. After the eProtocol radiation exposure edits are complete, your human research protocol will be reviewed by the Clinical Radiation Safety Committee (CRSCo). CRSCo approval is then communicated directly to the IRB.
To view the template risk language (according to radiation dose levels) used in the informed consent for the diagnostic use of ionizing radiation in human research, please click here.
Genomic Data Sharing
For guidance on the NIH Genomic Data Sharing Policy see the Stanford HRPP (Human Research Protection Program) Guidance (GUI-G1) document NIH Genomic Data Sharing for NIH Grant Submission.
You can access standard forms related to Institutional Certification Genetic/Genomic Data Sharing on the RMG website.