Research Policy Handbook
5.7
Training in the Protection of Human Subjects in Research
Policy Authority
Administrative Panel on the Use of Human Subjects in Medical Research
Policy Contact
Now in Policy Details
Presents requirements for training in the protection of human participants for any individuals at Stanford who are involved in research using human participants in either medical or nonmedical research.
1. Policy
Stanford University requires that all individuals working with human participants in either medical or nonmedical research complete an instructional program in the protection of human subjects. Training must be completed before the University will approve a protocol or release project funds. This requirement reflects the University's commitment to the protection of the rights and welfare of human participants in research, and incorporates the requirements of the National Institutes of Health (NIH).
At Stanford, the required tutorial is provided through the Collaborative Institutional Training Initiative (CITI). There is a course for nonmedical human subjects research, and a medical research course that covers both Human Subjects Research Training and Good Clinical Practices (below).
Upon review of the information presented at this site, individuals must verify their identify and maintain documentation of their completion of the course. Falsification in this regard is a violation of Stanford policy subject to appropriate corrective action.
2. Applicability
This policy applies to all individuals working with human participants under the auspices of Stanford, whether at Stanford facilities or at another location, and regardless of their institutional affiliation or source of funding. In the event that individuals from other institutions conduct research under the auspices of Stanford, they must complete the necessary training, but may do so at their home institution.
The training requirement applies to all faculty, staff, students, visitors, or any other individuals who work with human participants in research. Some examples include: individuals who obtain informed consent, administer surveys, or collect or analyze private or personal identifiable information from individuals. It is applicable for both medical and nonmedical research.
3. Implementation
The Protocol Director is responsible for assuring that all personnel working with human participants on the project complete the necessary training in accordance with the requirements listed below. An award will not be made unless the training is completed.
Certification of Human Subjects Training is required for all research projects involving human participants being conducted under the auspices of Stanford University regardless of the source of funding for the project. Where required by sponsoring agencies, the Protocol Director will need to obtain a Certification of Human Subjects Training form for the institutional representative to endorse and forward to the sponsor.
- NIH grants/contracts: When preparing a proposal for a project involving human participants, the Principal Investigator (PI) identifies key project personnel who are responsible for the design and conduct of the study. Per NIH Policy, for projects funded by the NIH, training in the protection of human research participants must be verified. The Certification of Human Subjects Training form is completed with the names of the key personnel that are involved with human subject research. The form is endorsed by the institutional official and must be provided to the agency before awarded funds will be released to Stanford University. A copy is also retained in the institutional file.
- All other non-NIH grants/contracts: For all non-NIH projects involving research on human participants, training is verified for listed personnel within eProtocol unless otherwise requested by the sponsor.
Subcontractors, consultants and other non-Stanford personnel must also complete this training. Key non-Stanford personnel must provide certification of human subjects training. They may satisfy this requirement at their home institutions provided that Stanford is assured of completion of the necessary training. A certificate confirming the training completed or a letter countersigned by a representative from the home institution will satisfy this requirement.
Members of Stanford's Administrative Panels for Human Subjects in either Medical or Nonmedical Research must complete initial and refresher Collaborative Institutional Training Initiative (CITI) courses.
Stanford University reserves the right to require additional training for researchers working with human participants where it is deemed to be necessary.
4. Good Clinical Practice (GCP) Training Requirement
All NIH-funded investigators and staff who are involved in the conduct, oversight, or management of clinical trials as defined by NIH must be trained in Good Clinical Practice (GCP), consistent with the principles of the International Conference of Harmonisation (ICH) E6 (R2).[1] As a result, the Institutional Review Board (IRB) will require assurance that GCP training will be completed. Your institutional official in RMG or OSR also will require verification that GCP training has been completed before sponsored project awards are issued. The Stanford medical research course covers both IRB Human Subjects Research Training and Good Clinical Practices (below). Awards will be withheld pending completion of GCP and human subjects (HS) training as required below:
Definitions
Investigator: The individual responsible for the conduct of the clinical trial at a trial site. If a clinical trial is conducted by a team of individuals at a trial site, the investigator is the responsible leader of the team and may be called the principal investigator.
Clinical trial staff includes: Individuals, identified by the investigator, who are responsible for study coordination, data collection and data management. The central focus of clinical trial staff is to manage participant recruitment and enrollment, to maintain consistent study implementation, data management, and to ensure integrity and compliance with regulatory and reporting requirements. These individuals may also seek informed consent from prospective participants, enroll and meet with research participants, and collect and record information from research participants. Clinical trial staff may also be called the research coordinator, study coordinator, research nurse, study nurse or sub-investigator.
Stanford University fully endorses the need for clinical research investigators and staff to meet international ethical and scientific quality standards for the design, conduct, recording, and reporting of research involving human participants. Therefore, Stanford University has determined GCP training will apply to all human subjects clinical trials regardless of funding source. This includes sponsored projects funded by external sponsors and non-sponsored projects funded with department or gift funding.
The Principal Investigator is responsible for identifying which staff are required to take the training and for ensuring the training is completed before participating in the study.
A. Decision Tree: NIH Definition of Clinical Trials
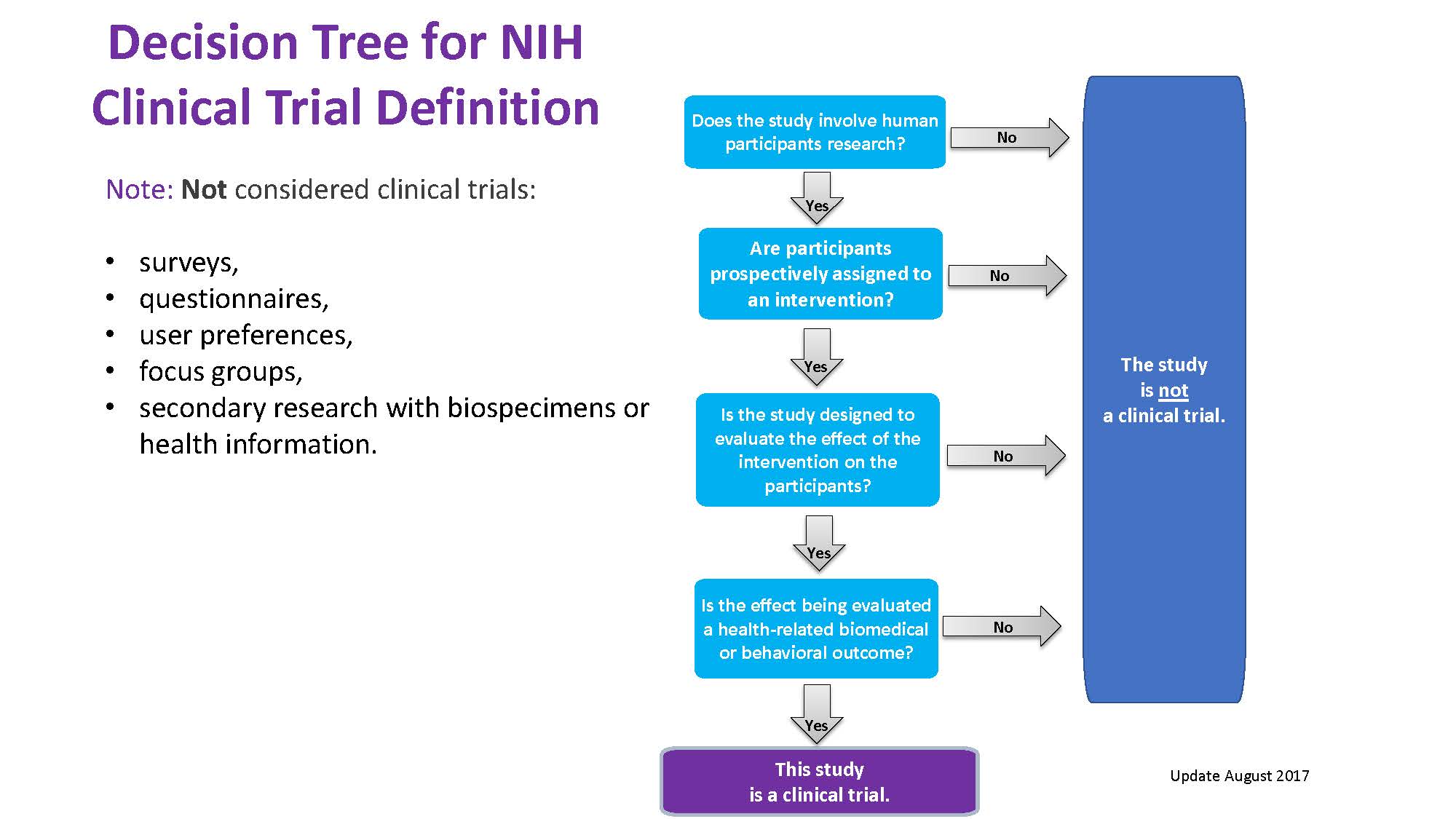
5. Good Clinical Practice (GCP) FAQs
Current Version: 03.18.24
Original Version: 10.01.00